
Onchocerciasis research
Since its foundation in 1974, TDR has worked with researchers and onchocerciasis control programmes to develop or improve approaches to effective onchocerciasis control and elimination. TDR was ‘the research arm’ of the Onchocerciasis Control Programme in West Africa (OCP, 1974–2002) as well as of the African Programme for Onchocerciasis Control (APOC, 1995–2015). Thus, TDR research priorities were identified not only based on the advice of its own external scientific advisory committees but also on the advice of the External Advisory Committee of the OCP and the Technical Consultative Committee of APOC. For many years, a bi-partite or tri-partite agreement between TDR, OCP and APOC provided for co-funding of agreed-upon research to be managed by TDR.
Overview of TDR funding for capacity building and research for onchocerciasis control
The figures below provide an estimate of TDR funding for research capacity building and research in terms of US dollars and the number of contracts from 1975 to 2011. Note that:
- Funding for the capacity building includes only grants for advanced degrees (Masters, PhD), short courses or specialized training directly addressing onchocerciasis research as well as grants specifically designed to address infrastructure needs of research sites. TDR also provided research capacity building and infrastructure building integrated into the research grants, which is consequently included under Research funding.
- Research funding is overestimated for some categories since some grants (e.g. for the establishment of assays to identify compounds potentially suitable for development as drugs against onchocerciasis) were not disease-specific, and for others is likely underestimated because for some grants the titles did not allow to establish whether they supported onchocerciasis research and in the early years the TDR grants data base did not include fields identifying the disease addressed.
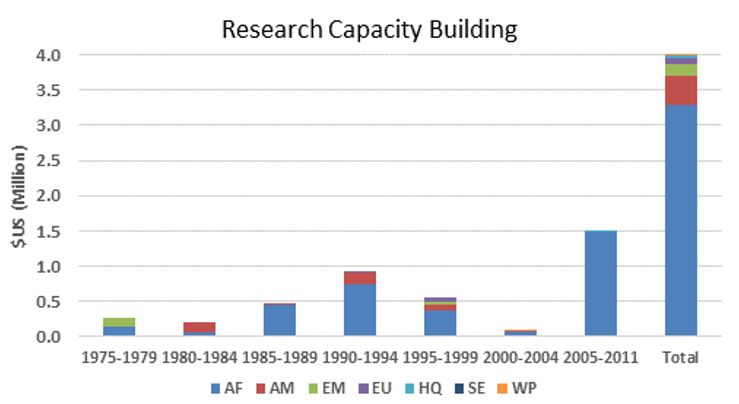
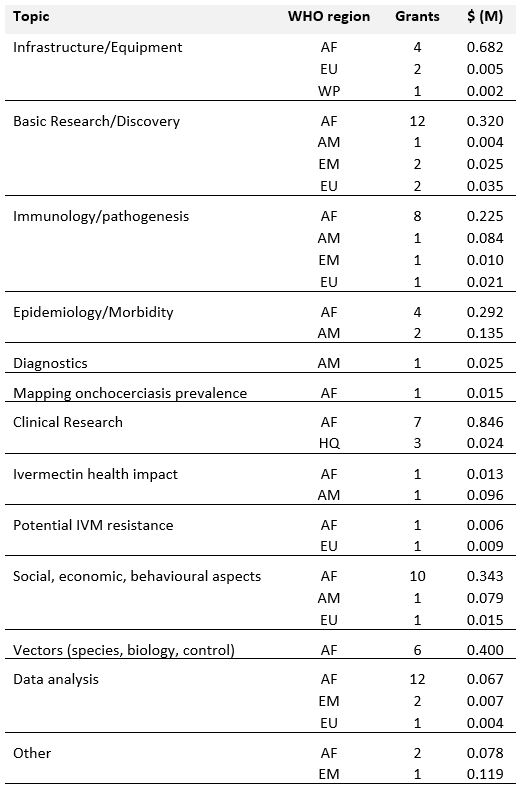
TDR funding in onchocerciasis research-related capacity building by WHO region
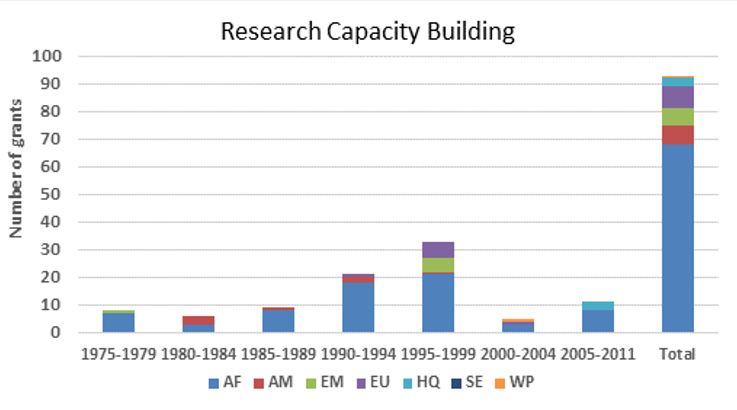
Grants for onchocerciasis research-related capacity building by WHO region
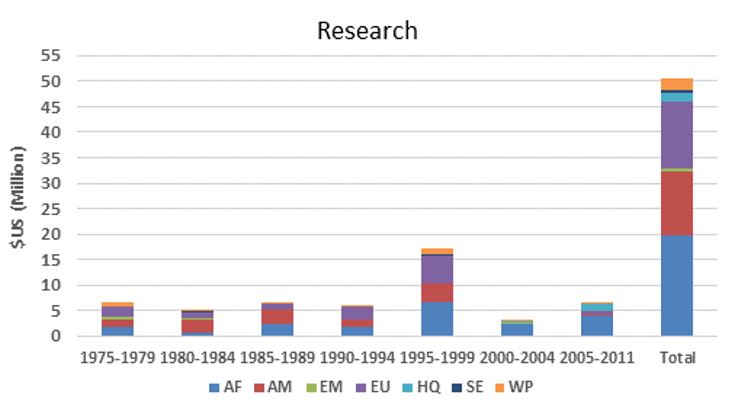
TDR funding for onchocerciasis research by WHO region
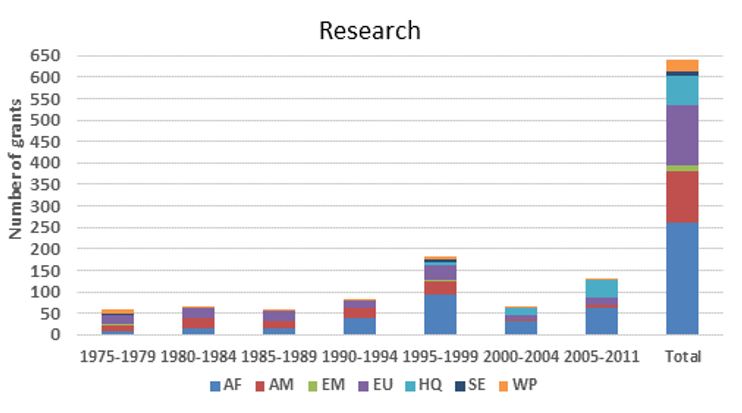
Grants awarded by TDR for onchocerciasis research by WHO region
AF: African Region, AM: Region of the Americas, EM: Eastern Mediterranean Region, EU: European Region, SE: South-East Asian Region, WP: Western Pacific Region.
HQ refers to costs incurred at TDR e.g. for meetings held in Geneva or purchases made by TDR for investigators.Overview of research capacity built by research area
The combination of the different types of capacity building (advanced degrees, short training courses on specific topics, including monitoring of clinical trials for compliance with Good Clinical Practice, provision of equipment and other infrastructure elements, renovation of buildings or even, for the Phase III study of moxidectin, construction of two new buildings) and research grant integrated capacity building) has resulted in research capacity that continues to serve the individuals, institutions and countries that received it.
The research capacity built at the Onchocerciasis Chemotherapy Research Center (OCRC) in Ghana resulted in research outputs acknowledged by external reviewers as important for the clinical development of ivermectin and provided the basis for the Phase II study of moxidectin.
For an overview of infrastructure capacity built for Phase II and III in Ghana, Liberia and DRC, see Related links.
The OCRC in Ghana has become part of UHAS, the University of Health and Allied Sciences, and continues research in support of onchocerciasis control and elimination, including further studies on moxidectin.
The Centre de Recherche pour les Maladies Tropicales (CRMT) in Ituri, DRC is now conducting two additional studies of moxidectin and passing on the training and experience to a new generation of physicians, nurses and laboratory technicians.
Related links
University of Health and Allied Sciences
African clinical trial site capacity available for other research
Overview of areas of TDR-funded research
The figures below show TDR funding in terms of US dollars and the number of contracts from 1975 to 2011 categorized broadly by "research area" across the whole period summarized (1975–2011) and by 5–7-year period. Note that (1) research funding is for some categories overestimated since some grants (e.g. for the establishment of assays to identify compounds potentially suitable for development as drugs against onchocerciasis) were not disease-specific, and for others likely underestimated because for some grants the titles did not allow to establish whether they supported onchocerciasis research and that (2) research into social, economic and behavioural aspects directly related to other research areas (e.g. community-directed interventions/CDTI) has been included under those areas.
The figures show the breadth of research questions addressed and the shift over time from research to improve the understanding of onchocerciasis and identification of potential drugs, diagnostics and vaccines to the research of the efficacy and safety of promising interventions, where and how to implement ivermectin mass drug administration and of the impact of community-directed treatment with ivermectin.
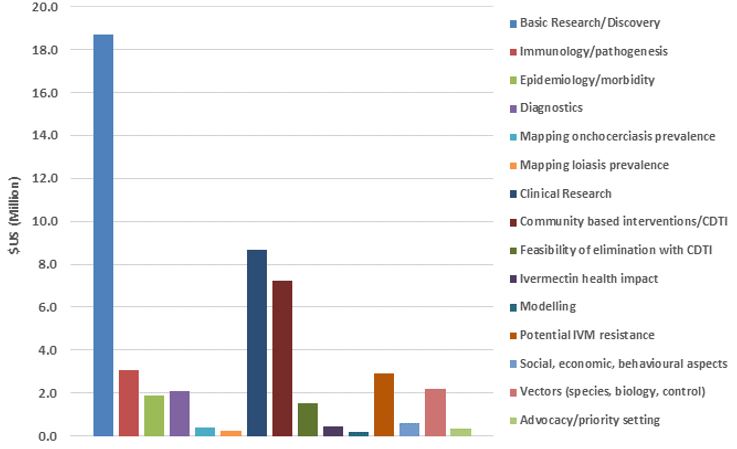
TDR funding from 1975 – 2011 by research area
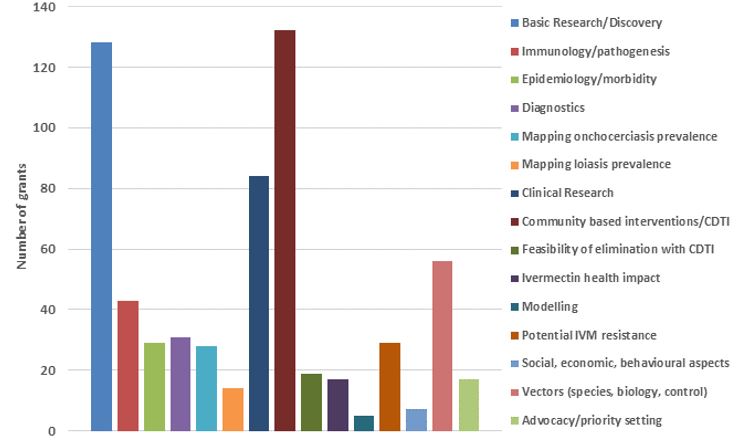
Research grants from 1975 – 2011 by research area
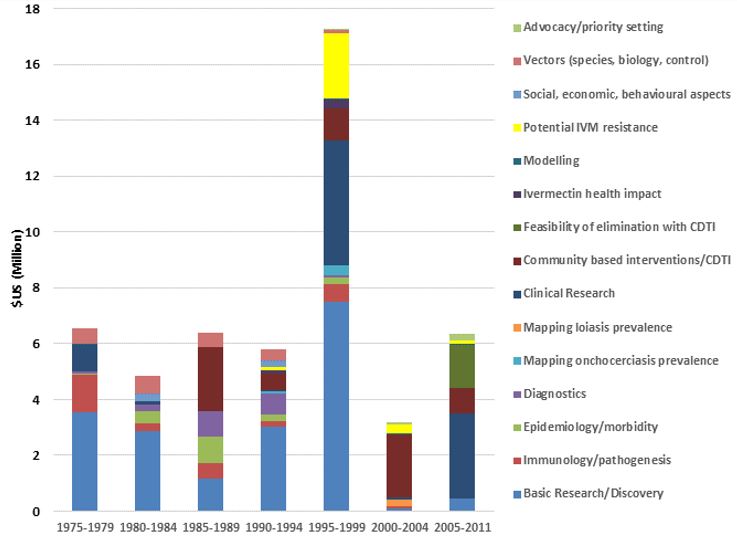
TDR funding by period and research area
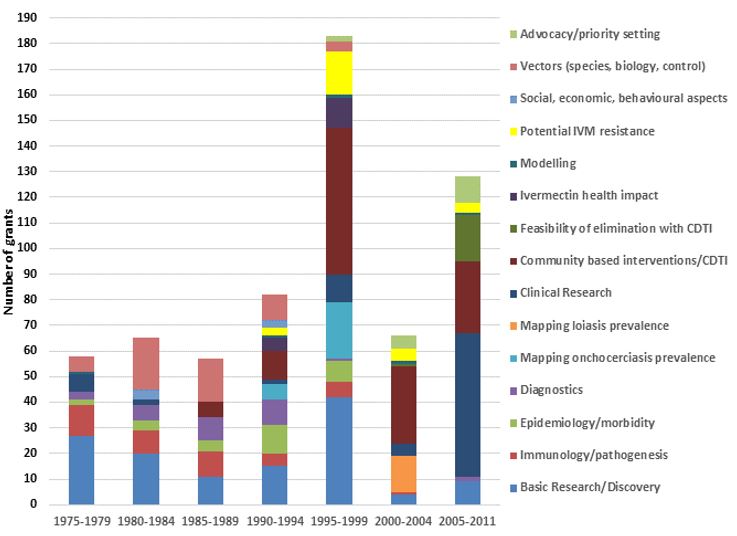
Research grants by period and research area
Most significant research outputs and outcomes
Research area | Most significant outputs | Most significant outcomes |
| Basic research/discovery | The establishment of in vitro and in vivo screening systems allowed the identification of ivermectin and moxidectin as potential anti-onchocercal drugs Efficacy of emodepside against O. volvulus macrofilariae ex vivo. | Initiation of clinical development of ivermectin (by Merck) and moxidectin (by TDR) Regulatory registration of ivermectin (without TDR support) and of moxidectin (with TDR support) Large scale studies to evaluate ivermectin and moxidectin efficacy and safety during Mass Drug Administration Initiation of further evaluation of emodepside by the Drugs for Neglected Diseases Initiative (DNDi) as a potential anti-onchocercal drug |
| Diagnostics | DNA probes to distinguish forest and savanna strains of O. volvulus and of O. volvulus and O. ochengi | Development of O-150 based pool screening, the method used for determining vector infectivity – the criterion for stopping interventions in WHO guidelines |
| Epidemiology/morbidity | Identification of the health and socioeconomic burden of skin disease in Central and Eastern Africa | Formation of the African Programme for Onchocerciasis Control (APOC) |
| Mapping onchocerciasis prevalence | Method for rapid epidemiological assessment and mapping of onchocerciasis prevalence | Identification of APOC areas for implementation of Community directed treatment with ivermectin (CDTI) |
| Mapping loiasis prevalence | Method for rapid epidemiological assessment and mapping of loiasis prevalence Correlation of Loa loa prevalence and microfilaraemia | Mapping of loiasis co-endemic areas where CDTI can only be rolled out only with a special strategy to minimize sequaelae of severe and/or serious adverse reactions |
| Clinical research | Network of onchocerciasis researchers facilitating Merck’s ability to develop a collaborative relationship for ivermectin clinical evaluation Methods for quantifying clinical reactions to microfilaricides Efficacy and safety data from phase II and III studies of moxidectin Data on the dependency of adverse reactions to ivermectin in Loa loa infected individuals on pre-treatment microfilaraemia Identification of individuals with lower than expected response to ivermectin (suboptimal responders) | Data on safety and efficacy of ivermectin supporting submissions for regulatory registration Data on safety and efficacy of moxidectin supporting submissions for regulatory registration Strategy for implementation of CDTI in onchocerciasis endemic areas depending on Loa loa prevalence. Further studies (not TDR funded) into the prevalence of suboptimal responders in areas with long term CDTI in Ghana and Cameroon. |
| Community based interventions/ CDTI | Safety of ivermectin during large scale use. Feasibility and effectiveness of community-directed treatment Ivermectin dosing by weight compared to height | Community directed treatment with ivermectin (CDTI) as the current standard strategy for onchocerciasis control and elimination Ivermectin dosing during CDTI based on height rather than weight |
| Feasibility of elimination with CDTI | Data showing that biannual or annual CDTI can reduce vector infectivity to the level WHO guidelines require for stopping CDTI | Change in APOC objectives from the control of onchocerciasis as a public health problem to the elimination of parasite transmission where feasible. |
| Ivermectin health impact | Effect of ivermectin treatment on eye and skin disease | Formation of the African Programme for Onchocerciasis Control (APOC) |
| Modelling | Enhancement of the transmission model ONCHOSIM with a module for adult form fecundity and the impact of drugs on fecundity | Contribution to the utility of ONCHOSIM for estimating the impact of drugs with effect on adult form fecundity (such as ivermectin) for estimating the effect of drug treatment on infection prevalence and intensity. |
| Vectors (species, biology, control) | Identification of Bacillus thuringiensis H-14 as a safe and effective biological insecticide with several commercial preparations of Bacillus thuringiensis H-14 registered Feasibility of vector elimination in Bioko Island | B.t. H-14 use by OCP for vector control from the time temephos resistance developed Additional insecticide for rotational larviciding to minimize the development of insecticide resistance APOC funded vector elimination in Bioko Island |
TDR publications on onchocerciasis research

Report on an informal meeting assessing the feasibility of initiating the first Phase II study of moxidectin...
WHO convened an informal meeting in Accra, Ghana to assess the feasibility of initiating the first study of moxidectin tablets in subjects infected with...

Eliminating River Blindness
The global effort to eliminate onchocerciasis (river blindness) is driven by close collaboration between research and public health agencies. Researchers...

The involvement of community-directed distributors of ivermectin in other health and development activities
Community-directed treatment with ivermectin (CDTI), through which millions of people are treated, is currently the principal drug delivery strategy for...

There is an urgent need to develop and scale up strategies that can ensure improved access of poor populations to existing, efficacious health interventions.One...
Guidelines for rapid assessment of loa loa
RAPLOA is a rapid assessment procedure for Loa loa that uses a simple questionnaire on the history of eye worm to predict whether or not loiasis is present...
Several cases of severe adverse reactions to ivermectin treatment have been reported from Cameroon in individuals with a high intensity of Loa loa infection....
Implementation and sustainability of community-directed treatment of onchocerciasis with ivermectin:...
This report describes the findings of a multi-country study that was designed to address the question of the long-term sustainability of community-directed...
Guidelines for analysis of REMO data using GIS
These guidelines were prepared following an informal consultation on the analysis and interpretation of Rapid Epidemiological Mapping of Onchocerciasis...
This report reviews the contributions made by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) to the development...
Community directed treatment with ivermectin
Onchocerciasis remains serious public health in large parts of tropical Africa where some 18 million people are affected. The most severe consequence of...
A manual for rapid epidemiological mapping of onchocerciasis
In this manual, a method of Rapid Epidemiological Mapping of Onchocerciasis (REMO) is described which is based on the selection of a very small sample...
(1990) During the past few years, there have been major advances in the development and use of microorganisms as microbial insecticides. Although several...

