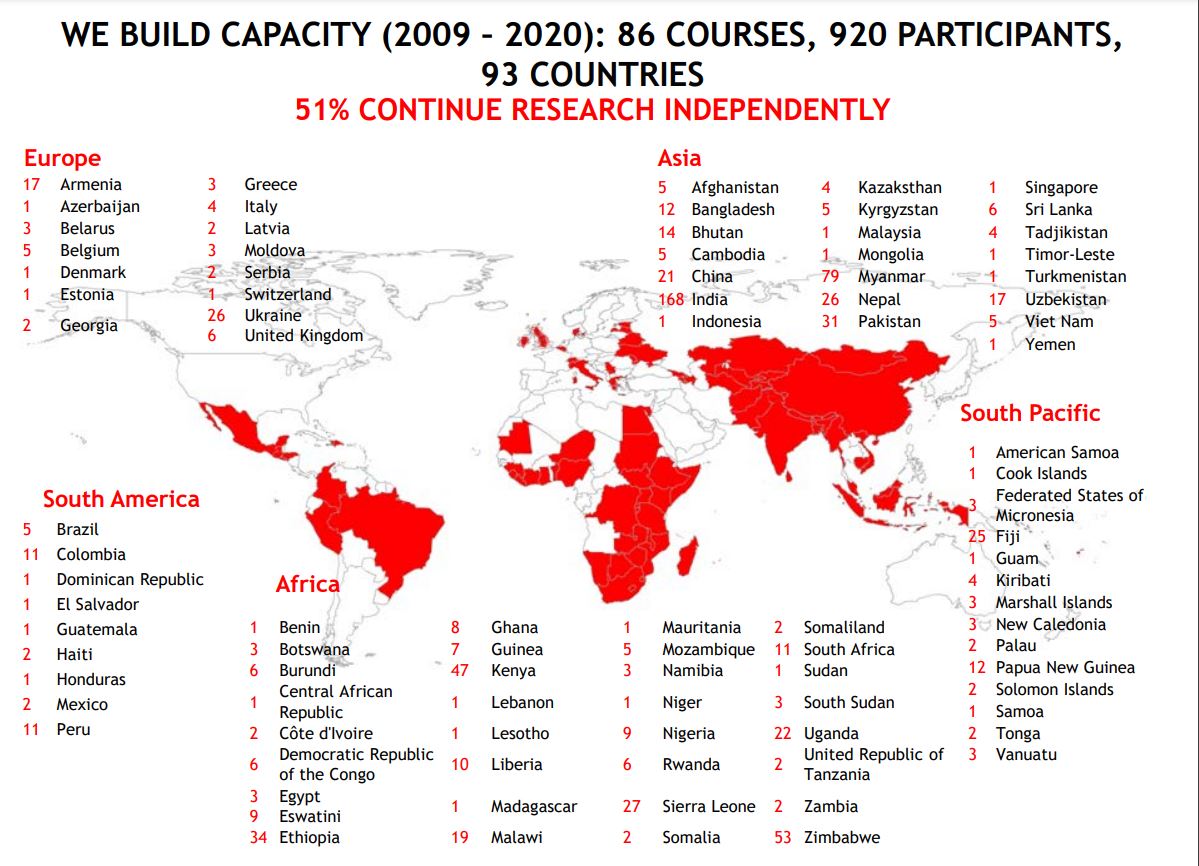
SORT IT targets implementers such as doctors, nurses, paramedical officers, data analysts, and programme officers, often with little or no prior research experience. Participants undergo training and conduct their research simultaneously. Each participant uses a relevant research project to learn the practical skills of how to write a study protocol, how to ensure quality-assured data capture and analysis, how to publish in a peer-reviewed journal and how to use the study findings to foster evidence-informed decision-making in public health. Participants must achieve milestones to move from one stage to the next and SORT IT courses are expected to achieve targets. Participants are supported with hands-on mentorship provided by experienced mentors.
Classic structure and milestones for a SORT IT course
- Module 1: Operational research and protocol development
- Milestone 1: Submission of protocol and the ethical review form within three weeks of completing module 1
- Module 2: Quality-assured data entry and analysis
- Milestone 2: Submission of the data documentation sheets within two weeks of completing module 2
- Milestone 3: Submission of proof of study completion and data collection about six weeks before module 3
- Module 3: Scientific manuscript writing
- Milestone 4 Submission of a paper to a peer-review journal within four weeks of end of module 3
- Module 4: Effective research communication for decision makers
- Milestone 5: Submission of a stakeholder map, evidence brief, power point presentations and elevator pitch within two weeks of completing module 4
Target scores for a SORT IT course
| Indicator | Target |
|---|
| Aggregate participant satisfaction score for each module | 80% |
| Participants complete all course milestones | 80% |
| Papers published within 12 months of submission | 80% |
| Papers assessed for effects of policy and practice within 15 months of submission | 80% |
The AMR- SORT IT project is geared to catalyze the evidence-to-action cycle from defining the most relevant research in countries to ensuring uptake of research findings. We train those who are embedded and retained within health systems and seek to enable the structures and processes needed for evidence-informed decision-making. SORT IT thus embraces the ‘Train, Embed, Retain and Enable’ strategy for individuals working within health systems. This approach is in line with WHO’s Thirteenth General Programme of Work, 2019–2023.
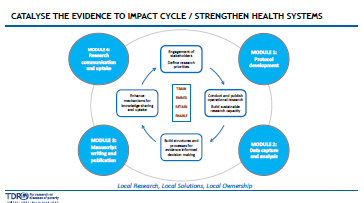
View SORT IT cycle
SORT IT is unique in being adaptable, output-oriented, striving for gender balance and having built-in metrics for accountability. It has been recognized by a DFID independent evaluation and by ESSENCE good practice guidelines. Several WHO documents refer to SORT IT including the World Health Report 2013, the Global action framework for TB research and the WHO European Action plan to strengthen the use of evidence for policy-making. Several NGOS and academic institutions have adopted the SORT IT approach.
You can expect to complete your research in about a year, develop a manuscript ready for submission to a peer-reviewed journal. SORT IT has a web-based alumni network that links up all SORT IT participants. Furthermore, alumni who are embedded within
disease control programmes and/or NGOs also have an opportunity to become operational research fellows and can pursue a PhD using their operational research work.
Participant eligibility criteria
Participant selection is guided by eligibility criteria which include the following:
- Must be actively involved in a disease control programme at the national, state, NGO, or health institution level.
- Outline a half-page of text that a) describes a relevant problem that the candidate has identified within a given programme and b) formulates a research question that is proposed to be developed into an operational research project. Please note that
research questions using routine programme data are preferred.
- Provide a written statement from the programme manager or relevant authority confirming the relevance of the research question and granting the applicant permission to have time and opportunity to carry out and publish his/her operational research.
- Must provide written commitment to attend all modules of SORT IT, return to their programme or institution after the course and implement course knowledge at programme level for a minimum of 18 months.
- Provide a written statement from a mentor (if available) or referee describing how the mentor knows the candidate and if he/she will be suitable for the course.
- If applicable, the ability to mobilize the funding required to carry out the operational research.
- Master of Public Health (MPH) or an equivalent, or a strong recommendation.
- Fluent in written and spoken English and/or the language of the course.
- Computer literate.
SORT IT is growing—expanding into new regions, addressing emerging public health priorities, adopting more complex study designs, and piloting innovative delivery methods. We actively promote partnerships and national leadership through SORT IT models.
You can support these tailored training opportunities in your country or institution by sponsoring a workshop in collaboration with TDR. Partner with TDR to host a SORT IT workshop that addresses global health challenges aligned with the TDR Strategy 2024-2029 , or help train new facilitators and bring world-class operational research capacity to your organization.
Criteria for SORT IT Courses and Use of Logos
1. TDR-led and/or funded (or co-funded) courses
These SORT IT programs fully comply with TDR standards and protocols. They carry both the TDR and SORT IT logos, reflecting official endorsement and funding.
2. Partner-led courses
These courses are organized and funded independently by partner institutions. They cannot be called SORT IT courses, as TDR does not oversee quality or take responsibility for these programs. Use of TDR or SORT IT logos is not permitted.
Applications for Funding of SORT IT Courses
Organizations seeking funding for SORT IT programs and aspiring to meet TDR–SORT IT standards should engage with TDR early. This ensures:
• Oversight of quality and adherence to standards
• Timely decisions on eligibility to use TDR and/or SORT IT logos
When WHO country offices or WHO collaborating centers are involved, engagement with TDR is mandatory, as TDR hosts the SORT IT Secretariat at WHO.