TDR’s Clinical Research and Development Fellowship (CRDF) programme has supported 19 fellows who have contributed to the development of the RTS,S malaria vaccine. On World Malaria Day, we take a closer look at the impact the fellowship programme has had on building African R&D capacity to tackle malaria and other infectious diseases in Africa.
It’s a testament to the success of TDR’s Clinical Research and Development Fellowship (CRDF) programme that 19 of its fellows were involved in the development of RTS,S/AS01 (RTS,S) – the antimalarial vaccine likely to save thousands of lives across Africa following WHO’s recent recommendation for its widespread use.
The vaccine targets the sporozoite stage of the malaria parasite and is the result of more than 30 years of research and development by GlaxoSmithKline (GSK). It was developed through a partnership with PATH, with support from a network of African research centres. Here we profile Dr Opokua Ofori-Anyinam from GSK and two of the CRDF fellows involved.
 Dr Opokua Ofori-Anyinam, Director of Clinical Development, GSK Biologicals (Belgium)
Dr Opokua Ofori-Anyinam, Director of Clinical Development, GSK Biologicals (Belgium)
An ambitious and visionary researcher of Ghanaian origin, Opokua played a pivotal role in setting up the capacity building partnership between TDR and GSK Biologicals (Belgium) and in training several fellows.
“It all began when we started early field efficacy trials of RTS,S in Africa,” said Opokua. “There weren’t many African scientists at the time who had the clinical/regulatory experience that we could collaborate with.” It’s through her efforts and the support of her senior colleagues and TDR staff that the partnership came into being.
The partnership started with co-funding and teaching in training programmes in Africa on Good Clinical Practice (GCP) and ethics and regulatory affairs for scientists and ethics committees, as well as providing support for African-based training organizations such as the African Malaria Network Trust and the Pan African Bioethics Initiative (PABIN). The capacity building programme then expanded to bringing mid-level African scientists to work at GSK Biologicals to gain further insight into vaccine development, as well as providing opportunities for the fellows to form networks with international researchers which would otherwise not have been available to them.
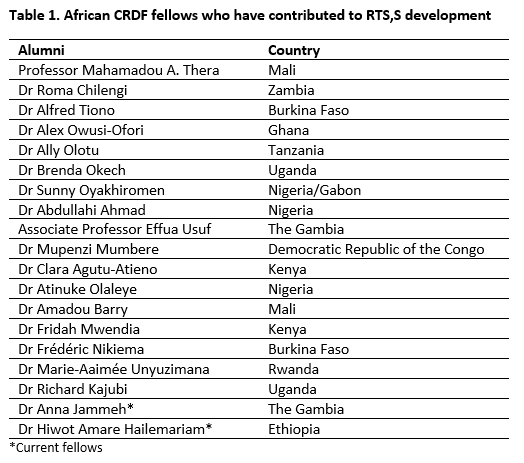
Since the programme’s establishment, at least 25 CRDF fellows have trained at GSK Biologicals, working on malaria and TB clinical development as well as pharmacovigilance of vaccines. Those involved in RTS,S development (see Table 1) worked on all three phases of clinical vaccine development as well as on ongoing pharmacovigilance.
Opokua points out the importance of the on-the-job training the fellows received: “They are not just there as observers. Fellows are equipped with a broad clinical and international regulatory and pharmacovigilance skillset that can be used for other things aside from malaria.”
GSK has been very transparent about why it is building research capacity. The hope is that fellows will go back to their home countries and train others, thereby building a corps of clinical researchers on the continent GSK and other companies can collaborate with. “It’s about research for Africa and for African diseases. It isn’t just about GSK,” said Opokua. “Everyone has gone on to do really great things, in Africa, as well as internationally,” she said.
 Professor Mahamadou Ali Thera, Professor of Parasitology-Mycology at the Malaria Research and Training Center, University of Sciences, Techniques and Technologies of Bamako, Mali
Professor Mahamadou Ali Thera, Professor of Parasitology-Mycology at the Malaria Research and Training Center, University of Sciences, Techniques and Technologies of Bamako, Mali
Mahamadou, who did his CRDF fellowship at GSK Biologicals (Belgium) back in 2000, worked on Phase I and Phase IIb studies for RTS,S in adults and children. “I was the very first fellow of this kind at GSK,” he points out, before describing how he worked with GSK staff and researchers at the MRC Unit The Gambia at LSHTM on the first proof of concept adult study which took place in the Gambia. Through his work and with the MRC team they were able to show that the RTS,S vaccine was efficacious and provided the evidence that allowed age de-escalation development into children. Later during his time at GSK he helped develop the first paediatric study protocol in African children.
“I was proud to have been part of the development of RTS,S,” he said, adding his belief that RTS,S development had lessons for all vaccine developers. “I doubt if there is anyone who can say they haven’t learnt something from it.”
Since his fellowship, Mahamadou has, together with other colleagues, established GCP training and the capacity for undertaking Phase I, II and III clinical trials of vaccines and drugs at his host institution. Now a professor, he has also gone on to work on two other promising blood-stage malaria candidate vaccines – AMA1 and MSP3.
 Dr Effua Usuf, Associate Professor and Clinical Epidemiologist, The MRC Unit The Gambia at LSHTM
Dr Effua Usuf, Associate Professor and Clinical Epidemiologist, The MRC Unit The Gambia at LSHTM
Now a senior scientist in the Gambia, Effua started her fellowship in May 2013, just after completing her PhD on pneumococcal vaccines and around the time that RTS,S was submitted to the European Medicines Agency for regulatory review.
As a CRDF fellow also based in GSK (Belgium), she split her time between clinical and epidemiological teams. As well as exploring vaccine schedules, her main focus was assessing the safety and immunogenicity of RTS,S in HIV-positive children and looking at the immune response to hepatitis B when RTS,S is co-administered with various vaccines. Following her fellowship, Effua has completed a TDR-sponsored project on vaccine wastage, and an MRC/LSHTM post-doctoral fellowship on pneumococcal carriage serotypes. Moving on, she conducted a similar vaccine wastage study in the Solomon Islands under a UNICEF consultancy, and she has contributed to the COVID-19 response in the Gambia.
As a female scientist with young children (the youngest only 4 years old at the time of her CRDF fellowship), she appreciated TDR’s flexibility and found working with women such as Opokua “really motivating.” Effua’s name would appear on more than five GSK publications, and as a leading African epidemiologist she was invited to give a presentation on her career at the World Health Assembly in 2017.
TDR’s Clinical Research and Development Fellowship (CRDF) allows early- to mid-career researchers in low- and middle-income countries to learn how to conduct clinical trials. Successful applicants are placed for 12 months in host training organizations (pharmaceutical companies, including members of the International Federation of Pharmaceutical Manufacturers & Associations, product development partnerships, or research organizations) and then receive a reintegration grant for 12 months at their home institution. The fellowship is implemented by TDR, the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. The fellowship is supported by the Bill & Melinda Gates Foundation.
For more information, please contact Dr Pascal Launois.

